
by Select Healthcare from NVS
The ingestion of unwanted substances in companion animals remains an important issue in practice.
- In 2020, 7.1 million pets fell ill after eating something toxic1
- In 2021, the Veterinary Poisons Information Service (VPIS) reported a 40% increase in the number of enquiries2
- The VPIS receives an average of 60 calls per day for assistance in cases involving suspected toxicity2
- Vets report seeing on average >3000 cases involving toxicity every week across the UK1
The most common toxins include:
- Human food (chocolate, grapes, raisins, xylitol, mycotoxins from mouldy food)
- Human medication and supplements (either over-the-counter or prescription)
- Veterinary medication (overdose)
- Plants
- Household chemicals
- RodenticidesInsecticides
- Herbicides and fertilisers (including bonemeal)
- Recreational drugs including vapes/e-cigarettes
In addition, many cases present where the offending toxin is unknown.
The chemical structures and behaviours of this expansive list of substances varies widely, including differences in their acidity/alkalinity, ionisation, polarity, solubility etc.
Management of toxic ingestion in pets
Alongside the use of emetics and gastric lavage (where not contra-indicated), antidotes (where available) and supportive and symptomatic therapies, adsorbents/binders are commonly administered.
Adsorbents
The objective of an adsorbent is to bind to the unwanted substance in the gut to help reduce the amount available for absorption into the system. The bound substance is then excreted in the faeces.
The adsorbents are administered orally either in a liquid or gel/paste formulation. If the animal has side effects from the substance that increases the risk of aspiration (sedation, breathing difficulties, neurological symptoms etc) then the adsorbent should either be administered under general anaesthetic post gastric lavage (where not contra-indicated), or administered via a nasogastric tube.
The substance ingested may either require a single or multiple administrations. When multiple administrations are given, they should be given every 4-6 hours for 24-48 hours depending on the substance.
This is because some drugs will undergo entero-hepatic recirculation, which presents more opportunities to capture the substance when it re-enters the gut through the bile and before it gets reabsorbed back into the system. The drugs may also be formulated as sustained-release, or delayed gastric emptying (e.g. opioids).
When using adsorbents in a repeated manner it is advisable to monitor the electrolyte status of the animal.
When is an adsorbent ingredient, not an absorbent?
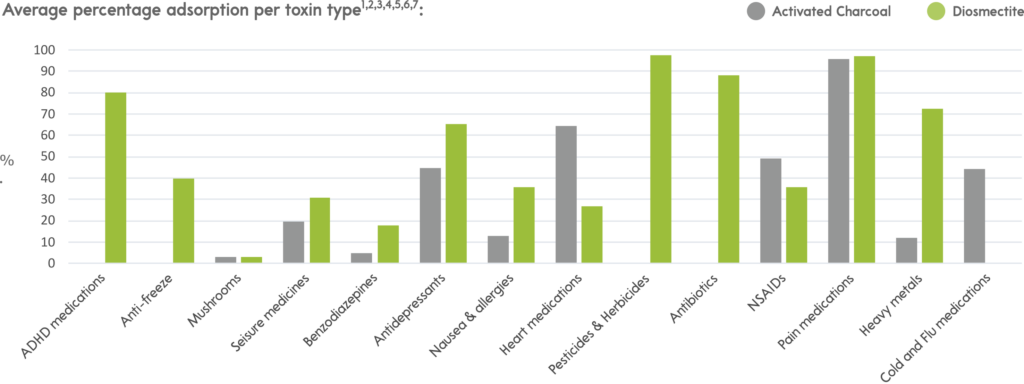
No adsorbent will bind to all toxins. The chemical properties of a substance dictate its binding affinity for a given adsorbent. The most common adsorbent administered is Activated Charcoal (AC).
However, many commonly presented unwanted substances have little or no affinity to activated charcoal as their chemical properties do not match those it has a particular affinity for (listed to the right), so relying on activated charcoal alone creates a management gap.
So, what can be used to help close this gap? The answer is Diosmectite.
The percentage absorption of some commonly presented substances and how they differ between the two can be observed in the graph below.
Activated charcoal

Activated charcoal is made from a carbon-based source that is heated to very high temperatures, followed by the erosion of parts of the structure with steam. This creates numerous pores and vastly increases the surface area available for binding.
Activated Charcoal has a particular affinity for substances that are:
- Acidic substances
- Non-ionised substances
- Poorly water-soluble substances
- Non-polar organic molecules
- Anionic particles
- Non-Specific adsorption
Diosmectite
- Alkaline or basic substances
- Hydrophilic substances
- Cationic compounds
- Amphoteric compounds
- Non-ionic compounds
- Organic solutes
- Specific adsorption
Rather than having to choose between 2 different adsorbents, it could be beneficial to have one product that contains both ingredients. In addition, it is worth remembering that often the particular substance is unknown and there are some substances where both adsorbents have a binding effect together (e.g chocolate, many pain medications).
Introducing DuoTox

Duotox is a broad-spectrum toxin binder containing a combination of activated charcoal and diosmectite. It also contains:
Sorbitol: Acts as a laxative by retaining water in the large intestine through osmotic pressure, stimulating peristalsis, and exerting diuretic, laxative, and cathartic effects.
Glycerine: Functions as a cathartic, causing rectal distension and irritation, which increases the urge to defecate and decreases GIT transit time, reducing systemic toxin absorption.
Curcumin: Provides renal and liver protection by reducing the expression of tight-junction proteins, thereby suppressing proinflammatory cytokines. It also aids in the reduction of oxidative stress within the liver.
Piperine: Enhances curcumin absorption, thereby improving its effectiveness in scavenging free radicals and downregulating inflammatory mediators.
Administration of adsorbants
Administering adsorbents can be challenging. The best formulation depends on the animal’s condition. For instance, a liquid formulation is ideal for post-gastric lavage or nasogastric tube administration, while a gel or paste is suitable when there are no contraindications or as a follow-up to the initial dose.
Liquid suspensions often form sediment at the bottom of the bottle, making it difficult to reconstitute fully. This can lead to the administration of inconsistent dosages.
Pastes and gels, on the other hand, can also sometimes be tricky, especially with large syringes that require one-handed operation while opening the animal’s mouth with the other. High pressure is also often needed to plunge the syringe, complicating administration further.
Finally, many products are often lacking in palatability.
DuoTox: Designed with use in mind

DuoTox is available in both a liquid suspension and a gel formulation.
DuoTox is formulated for consistency, ensuring no sedimentation of the liquid, (give a quick shake before use). Its maximum concentration allows for ease of use by minimising volume, while the smooth flow of both liquid and gel aids application and reduces the risk of aspiration. Additionally, DuoTox features an enhanced palatability profile.
Duotox Liquid
-
Soft, squeezy bottle and bendy nozzle to aid administration
-
Nozzle fits directly to nasogastric tubes
Duotox Gel
-
Dial-a-dose syringe for easy administration
-
Wide nozzle bore for ease of flow
-
Reduces pressure needed on plunger
-
Reduces risk of blocking and sudden product release
-
Up to 75% wider than other products
Adsorb-Eliminate-Support
For peace of mind when you don’t know what they’ve been up to.
References
- directlinegroup.co.uk/en/news/brand-news/2021/7-1-million-pets-fall-ill-after-eating-something-poisonous.html
- vpisglobal.com/wp-content/uploads/2023/11/VPIS_3006-Annual-Report-2022.pdf
- Albengres, E, Urien, S, Tillement, J.P., Oury, P., Decour, S, Flouvat, B., and Drieu, K. (1985) Interactions between Smectite, a mucus stabilizer and acidic and basic drugs. European Joumal of Clinical Pharmacology, 28:601-605.
- McGinity, J, and Loch, J. (1976) In vitro adsorption of various pharmaceuticals to montmorillonite, Journal of Pharmaceutical Sciences. 65:6.
- Neuvonen, P.J. and Olkola, K. 198) Oral activated charcoal in the treatment of intoxications, Role of single and repeated doses: Medical Toxicology, 3:33-58. Doi. 012-5966/88/0001-0033.
- American Academy of Clinical Toxicology and Equropean Association of Poisons Centres and Clinical Toxicologists. (2005) Position paper: Single dose activated charcoal: Clinical toxicology, 43:61 -87: Doi: 10.1081/CLT-20051867.
- Zeliner, T., Proso, D., Forber, E., Hoffmann-Walbeck, P., Genser, D, and Eyer, F. (2019) The use of activated charcoal to treat intoxications. Deutsches Arzteblatt International, 116:311-117. Doi: 10.3238/arztebl2019.0311.
- Castela-Popin, N., Cai, S., Vatier, J,, Keller, F., Souleau, C.H. and Farinotti, R. (1999) Drug interactions with diosmectite: a study using the artificial stomach-duodenum model. Intérnational Joumal of Pharmaceutics, 182:111 – 119.
- Frissel, M.J. (1961) The adsorption of some organic compounds, especially herbicides, on clay minerals. Versi. Landbouwk. Onderz., 67-3.
The article was originally posted in The Cube magazine, June 2024 issue. Click here to read the magazine.



